top of page



1/3
Soft Materials Research Lab
The Sharma Group
Low pH self-organisation of similarly charged Polyethylenimine chains
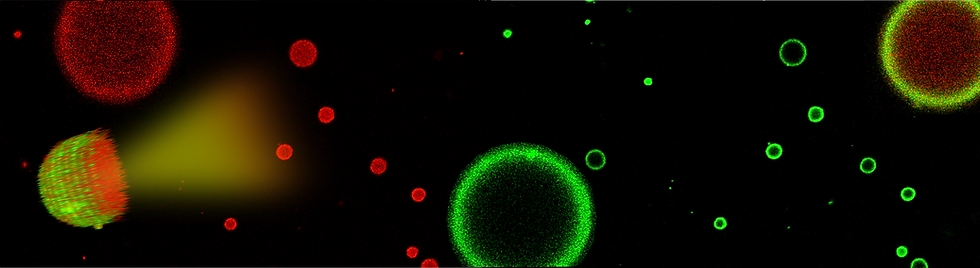
Polyethyleneimine (PEI), an amine containing weak polyelectrolyte has emerged as a fascinating candidate for designing advanced materials with tailored properties and functionalities due to the presence of versatile interactions in it. It has the tendency to form polyplexes by electrostatically binding with oppositely charged small molecules or macromolecules, such as nucleic acids (DNA and RNA). These polyplexes find applications in gene transfection or drug delivery. The main driving force behind PEI's potential lies in its highly charged nature, resulting from the presence of primary, secondary, and tertiary amines. However, all amines do not become fully protonated even when the pH of the PEI solution is lowered to 1. This distinctive behavior leads to PEI's remarkable buffering capacity, referred to as the "proton sponge" effect, that makes it the gold standard candidate for non-viral gene transfection. Our research has unveiled truly unprecedented assembly of 70% protonated PEI chains into hollow, robust fibrillar network at pH around 3. Further, by sparsely incorporating dyes such as Fluorescein Isothiocyanate (FITC) or Rhodamine Isothiocyanate (RITC) into branched PEI chains, we have demonstrated their spontaneous assembly into fibrillar and sheet-like structures. This phenomenon can be primarily attributed to a combination of proton-sponge effect of PEI chains and excited state proton loss from the covalently attached fluorophores. Through the use of specific wavelengths of light, dye-polymer interactions are initiated, leading to the creation of structures resembling bead necklaces in fibers or the collapsing of sheets.
 |  |
|---|
bottom of page